Affiliate Investigators from diverse disciplines and over 20 UW Departments utilize NORC services in the conduct of ground-breaking science in areas ranging from methods development and molecular biology to public policy and communication-based interventions.
Overview
Affiliate investigators enjoy priority access to all UW NORC core facilities and services. Through this network, Affiliate Investigators will also gain exposure for their research and publications, have an opportunity to network and collaborate with other UW NORC investigators, and will have expanded access to nutrition and obesity-related conferences, seminars, trainings, and funding opportunities.
The UW NORC offers two categories of membership – Affiliate Investigator (AI) and Associate Affiliate Investigator (Associate AI). The benefits are described in the table below.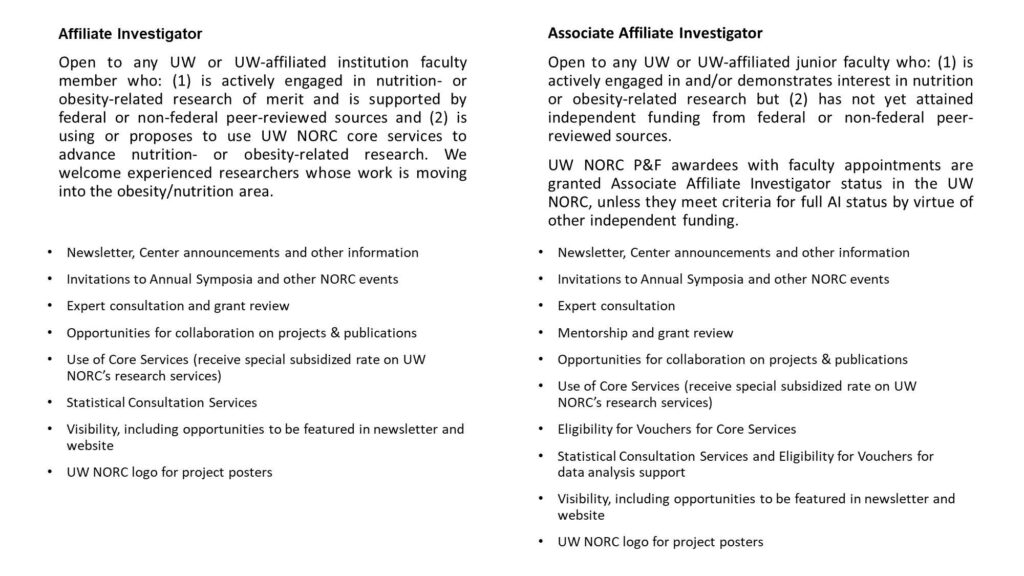
Apply below to become an Affiliate Investigator or Associate Affiliate Investigator!
If you are with the University of Washington (requires UWNetID to log-in):
If you are with an UW-affiliated institution (e.g. Fred Hutch, Veteran Affairs, Seattle Children’s, etc.):
Current Affiliate Investigators

Michael Ailion, PhD

Kim Alonge, PhD

Alessandro Bitto, PhD

Pavan Bhatraju, MD, MSc

J. Ernie Blevins, PhD

Karin Bornfeldt, PhD

Michael Bruchas, PhD

Linda Buck, PhD

Carlos Campos, PhD

Rotonya Carr, MD

Vincenzo Cirulli, MD, PhD

Paul Crane, MD, MPH

Julia Cui, PhD

Ian de Boer, MD, MS

Laura den Hartigh, PhD

Mauricio Dorfman, PhD

Nicole Ehrhardt, MD

Kendra Francis, MD
Associate AI

Amanda Fretts, PhD

Jose Garcia, MD, PhD

Sean Gibbons, PhD

Heather Greenlee, ND, PhD, MPH

William Hagopian, MD, PhD

Angela Hanson, MD

Renee Heffron, PhD, MPH

Margaret Heitkemper, PhD, RN

D. Taylor Hendrixson, MD FAAP

Andrew Hoofnagle, MD, PhD

Alyssa Huang, MD

Rebecca Hull, PhD

James Hurley, PhD

Brian Iritani, DVM, PhD

Nina Isoherranen, PhD

Jessica Jones-Smith, PhD, MPH, BS

Matthew Kaeberlein, PhD

Steven Kahn, MB, ChB

Kendra Kamp, PhD, MS

Alice Kane, PhD

Jenny Kanter, PhD

Bryan Kestenbaum, MD

Francis Kim, MD

Meghan Koch, PhD

Johanna Lampe, PhD, RD

Cecilia Lee, MD, MS

Rozenn Lemaitre, PhD, MPH

Ben Lidgard, MD

Kate Markey, PhD, MBBS

Jason Mendoza, MD, MPH

Stephen Mooney, PhD

Gregory Morton, PhD

Charles Muller, PhD

Marian Neuhouser, PhD, RD

Grant O'Keefe, MD, MPH

Vitor Oliveira, PhD

Stephanie Page, MD, PhD

Jisun Paik, PhD, RD

Richard Palmiter, PhD
Devasena Ponnalagu, PhD, MS
Associate AI

Peter Rabinovitch, MD, PhD

Dan Raftery, PhD

Christian Roth, MD

Brian Saelens, PhD

Jarrad Scarlett, MD, PhD

Jeannette Schenk, PhD, MS, RD
Associate AI

Ellen Schur, MD, MS

Michael Schwartz, MD

Leticia Sewaybricker, MD, PhD

Marta Soden, PhD

Garret Stuber, PhD

Chongren Tang, PhD

Joshua Thaler, MD, PhD

Arthi Thirumalai, MD
Associate AI

Rong Tian, MD, PhD

Jenny Tong, MD, MPH

Subbulaxmi Trikudanathan, MD, MRCP, MMSc

Kristina Utzschneider, MD

Tomas Vaisar, PhD

Russell Van Gelder, MD, PhD

Luke Wander, MD

Joanne Wang, PhD

Hunter Wessells, MD

Oleg Zaslavsky, PhD, MHA, RN

Sakeneh Zraika, PhD


